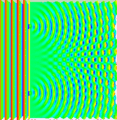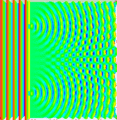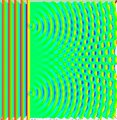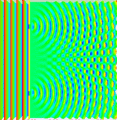
Last time covered how sometimes light behaves like a particle and others like a wave along with how the double-slit experiment was used to demonstrate these properties. For example, if a steady light comprised of numerous individual photons hit a plate with one tiny slit to allow them through, rather than getting a line that matched the slit on the opposing wall it would be spread out in a pattern that was concentrated toward the center and fuzzy around the edges. (See picture below.)
When they used a plate that had two slits a single photon would leave a dot, as expected, but by continuing to release them one at a time they would eventually form an interference pattern, the same as what resulted from a steady light source. It was as if each photon had a mind of its own yet collectively they would arrange themselves in a certain pattern. While exactly where each photon would arrive couldn’t be predicted, the pattern itself could be, based on the wavelength of the light. Thus there was a certain probability that a photon would arrive in a certain place, some more than others, but which exact one would go where was unknown.
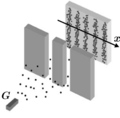
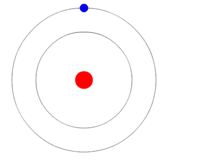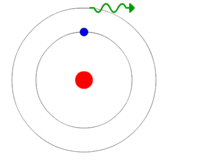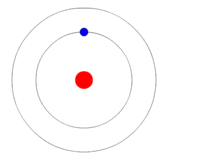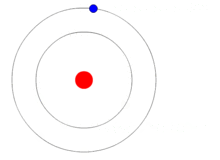
It was apparent they couldn’t predict exactly where a single photon would land but if it was a discrete particle of light then it followed that it would go through one slit or the other. (Remember that the interference pattern resulted because there were two slits so the waves could overlap.) Thus, scientists, the first of whom was Thomas Young (1773-1829), decided to find out which slit of the two choices each photon went through. To do so they polarized the light going through each slit in a different way with the detector on the other side capable of telling the difference. The photon could still theoretically “choose” which slit (or both) it would go through, but they would be able to tell which one by its polarization when it arrived on the detector.
Sneaky. But outsmarting Mother Nature is not an easy task.
Much to their surprise, when they sent one photon at a time toward the slits where it was polarized the interference pattern did not emerge!
Whoa!
Instead, they got random spots of light which indicated individual particles. Polarizing the light did not destroy its ability to build interference patterns so this didn’t make sense. The results implied that when they set things up so that they’d know whether the photon went through one slit or the other that the individual photons lost their right to choose and behaved like a particle. In other words, the probability wave function had collapsed when the final result would be determined.

In other words, the photon can change from a wave to a particle when someone is trying to figure out exactly what it’s going to do. When someone is watching, it behaves like a particle that not only goes through one opening or the other but loses its wave properties as well.
Say what?
Back then the expression WTF? didn’t exist yet, but something along those lines was definitely what was going through numerous scientific minds. By all appearances, if someone was watching, i.e. measuring the outcome, then the probability wave collapsed and the photons acted like particles.
Thinking perhaps this was because they were polarizing the photons before they went through one slit or the other, even though they knew that didn’t stop the light from forming an interference pattern, they rigged things up to determine which slit it had gone through afterwards. Much to their surprise they got the same result as before, a rain of itinerant particles, as if each photon had either known in advance or perhaps even went back in time, deciding how to behave.
This introduced the concept of an observer affecting the outcome. Suddenly consciousness was part of the mix, or at least seemed to be since there was no other explanation. Of course physicists who deal exclusively with the physical world were less than enchanted by all this woo-woo stuff. Thus began the philosophical notion of whether or not a tree that fell in the forest made a sound if no one was there to hear it. May I remind you that these are very intelligent people we’re dealing with here and while some of them may not be wrapped to tight as they walk the genius-insanity interface; nonetheless, they are a whole lot smarter than the rest of us.
Einstein called this “spooky action at a distance” and didn’t believe it, even though he was the one who theorized that energy and matter were essentially the same as expressed by his famous equation E=mc2. To this day people are still arguing about this aspect of quantum theory with different conclusions. Is it possible that an observer or some form of consciousness can influence physical matter? Do we, indeed, create our own reality?
What do you think?
(Diagrams courtesy of Wikipedia Commons)